Contact person: Jiří Henych
We prepare various nanomaterials, including nanoscale semiconductor oxides (e.g., TiO2, CeO2) and their nanocomposites, molecular clusters of transition metals and others to exploit their photocatalytic properties. Specifically, we focus on the solar- and visible light-induced photodegradation of emerging pollutants in water and volatile compounds (mainly indoor air pollutants) in air. Many of the materials we developed can also be used for bacteria and virus inactivation or preparation of self-cleaning surfaces.
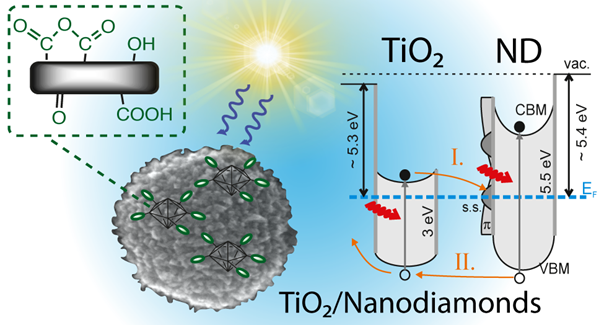
Figure 1: Composite photocatalyst based on TiO2/Nanodiamonds with simplified scheme of band energy levels and charge transfer process.
We mainly use low-temperature synthesis in water to prepare highly active nanostructured transition metal oxides, which we can prepare on a larger scale (hundreds of grams to grams).
We also prepare completely new nanocomposite materials using metal oxide nanoparticles and low-dimensional carbon nanomaterials (such as graphene, graphene oxide, nanodiamonds, Fig. 1 and 2), molecular clusters or other photosensitizers and materials. We then study in detail interactions and behavior at the interface, synergistic effects with the aim of improving the photophysical and photochemical properties of individual materials, their adsorption properties, stability and other useful properties that are essential for real applications of photocatalytic materials.
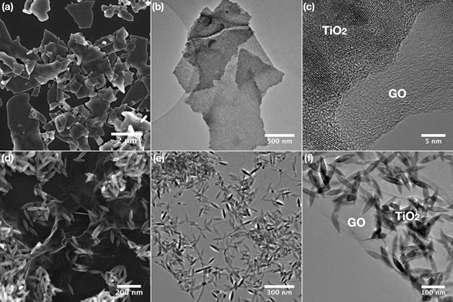
Figure 2: Transmission electron microscopy (TEM) images of the photocatalysts based on TiO2 nanoparticles deposited on graphene oxide nanosheets.
We study the mechanism and kinetics of decomposition of real water pollutants (e.g., drug residues, pesticides, industrial chemicals) using HPLC with DAD and MS detection.
The phenomena at the gas-solid interface we study in detail by using a home-made experimental setup (Fig. 3) based on operando/in situ DRIFT spectroscopy with Praying MantisTM reaction chamber. We can thus study adsorption, surface chemistry, and light-induced reactions on real pollutants such as formaldehyde, methanol, organophosphates, and other small organic molecules.

Figure 3: Operando DRIFTS setup for studying photocatalytic degradation reactions on the gas-solid interface.
References:
- M. Šťastný, D. Bavol, J. Tolasz, P. Bezdička, J. Čundrle, M. Kormunda, I. Dimitrov, P. Janoš, K. Kirakci, J. Henych. Interfacial behavior of ceria grown on graphene oxide and its use for hydrolytic and photocatalytic decomposition of bisphenols A, S, and F. Environ. Sci.:Nano 2024, 12, 502-513.
- J. Henych, A. Mattsson, J. Tolasz, V. Štengl, L. Österlund. Solar light decomposition of warfare agent simulant DMMP on TiO2/graphene oxide nanocomposites. Catal. Sci. Technol. 2019, 9, 1816-1824.
- J. Henych, Š. Stehlík, K. Mazanec, J. Tolasz, J. Čermák, B. Rezek, A. Mattsson, L. Österlund. Reactive adsorption and photodegradation of soman and dimethyl methylphosphonate on TiO2/nanodiamond composites. Appl. Catal. B-Environ. 2019, 259, 118097.