Contact person: Jan Šubrt, Petra Ecorchard, Monika Motlochová
Titanic acid is a general name for a family of chemical compounds of the elements titanium, hydrogen, and oxygen, with the general formula [TiOx(OH)4−2x]n. Various simple titanic acids have been claimed, mainly in the older literature. However, at present no crystallographic and little spectroscopic support exists for these materials.
Metatitanic acid (H2TiO3) is an important intermediate in the production of titanium dioxide by the sulphate process. High sorption capacity of this material for various cations and anions is known, but some of its properties, as poor filterability and formation of stable colloid in an aqueous medium, prevent its wider use. A new preparation method for the synthesis of TiO2 microrods in aqueous media starting with solid hydrated titanyl sulfate crystals with defined morphology is presented. The method is based on the extraction of sulfate ions from the crystals and their replacement with hydroxyl groups in aqueous ammonia solution leaving the Ti−O framework intact. The particle size and morphology of the starting hydrated titanyl sulfate is closely preserved in the pseudomorphs of amorphous metatitanic acid including such details like the layered structure of the original hydrated titanyl sulfate crystals. This material removes all drawbacks of metatitanic acid for practical use as a sorbent while maintaining high sorption capacity. This material exhibits high sorption capacity for both radioactive ions (Cs+, Sr2+, Co2+, Eu3+, UO2 and heavy metals (Pb2+, Cd2+, etc.), as well as for some organic cations (amines).
Large scale of zeolites is already known for several years. Anyway, it was found that zeolites are very good sorbents of radionuclides. Therefore, the research of radionuclides sorbents and sorbents of heavy metals was extended to zeolites and modified zeolites and will be further studied.
References
Motlochova M., Slovak V., Plizingrova E., Lidin S., Subrt J., Highly-efficient removal of Pb(ii), Cu(ii) and Cd(ii) from water by novel lithium, sodium and potassium titanate reusable microrods, RSC Advances, 10 (2020) 3694-3704. IF=3,070
M. Motlochova, V. Slovák, E. Pližingrová, L. Szatmáry, P. Bezdička, J. Šubrt, The influence of annealing temperature on properties of TiO based materials as adsorbents of radionuclides, Thermochimica Acta, (2019). IF=2.189, Q2
M. Bubenikova, P. Ecorchard, L. Szatmary, O. Mrozek, P. Salacova, J. Tolasz, Sorption of Sr(II) onto nanocomposites of graphene oxide-polymeric matrix, Journal of Radioanalytical and Nuclear Chemistry, 315 (2018) 263-272. IF=1,181, Q2
M. Motlochova, V. Slovak, E. Plizingrova, M. Klementova, P. Bezdicka, J. Subrt, Thermal decomposition study of nanostructured amorphous lithium, sodium and potassium metatitanates, Thermochimica Acta, 670 (2018) 148-154. IF=2.189, Q2
M. Klementova, M. Motlochova, J. Bohacek, J. Kupcik, L. Palatinus, E. Plizingrova, L. Szatmary, J. Subrt, Metatitanic Acid Pseudomorphs after Titanyl Sulfates: Nanostructured Sorbents and Precursors for Crystalline Titania with Desired Particle Size and Shape, Crystal Growth & Design, 17 (2017) 6762-6769. IF=3.972 , Q1
Gallery
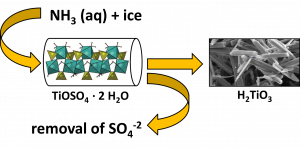
Synthesis of metatitanic acid
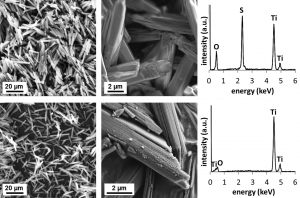
Comparsion of morphology of default titanylsulphate monohydrate (up) and metatitanic acid (down)
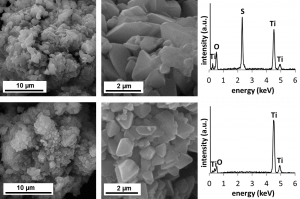
Comparsion of morphology of default titanylsulphate dihydrate (up) and metatitanic acid (down)
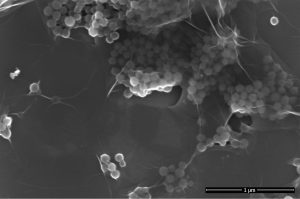
Nanocomposite of polystyrene with graphene oxide

SEM and STEM images of nanocomposites of polyamide with graphene oxide

Zeolite of type P