Contact persons: Kamil Lang, Kaplan Kirakci
We investigate new cluster complexes (e.g., Mo, W, Re, Cu) as biomaterials with photodynamic and radiosensitizing properties (Fig. 1). We were the first to propose that such cluster complexes constitute versatile theranostic tools with desirable features such as high luminescence quantum yields, production of singlet oxygen, and radioluminescent properties attractive for X-ray luminescence computed tomography or X-ray induced photodynamic therapy of cancer.
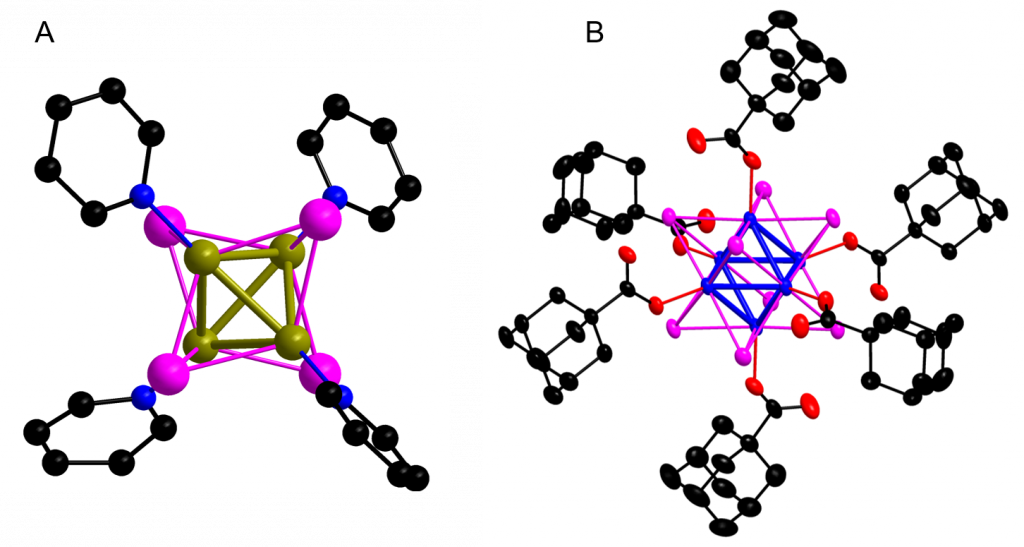
Figure 1. (A) Typical structure of Cu(I) clusters: Cu4I4(pyridin)4. Color coding: Cu (dark yellow), I (magenta), N (blue), C (black), hydrogen atoms are omitted for simplicity. (B) Typical structure of octahedral molybdenum clusters with adamantane ligands: [Mo6I8(adamantan)6]2-. Color coding: Mo (blue), I (magenta), O (red), C (black), hydrogen atoms are omitted for clarity.
Singlet oxygen
Singlet oxygen O2(1Dg) is a potent mediator of phototoxicity and is typically generated by energy transfer from the excited triplet states of a photosensitizer to molecular oxygen (Fig. 2). This feature, coupled with the limited diffusion length of the O2(1Dg) in cells (d ~ 150-190 nm) due to its short lifetime, leads to a high selectivity in the destruction of targeted problematic cells. In addition, O2(1Dg) has strong bactericidal and virucidal properties, which are the bases for a promising method for fighting microorganisms (often resistant) – antimicrobial photodynamic inactivation. Singlet oxygen interacts with cell structures and interferes with different metabolic pathways, preventing the development of resistance of microorganisms towards photodynamic treatment.
Selected references:
Lang, J. Mosinger, D. M. Wagnerová: Photophysical properties of porphyrinoid sensitizers noncovalently bound to host molecules; models for photodynamic therapy. Coord. Chem. Rev. 248/3-4 (2004) 321-350.
Lang, J. Mosinger, P. Kubát: Nanofibers and nanocomposite films for singlet oxygen-based applications. Chapter 15, pp. 305 – 321. Singlet Oxygen: Applications in Biosciences and Nanosciences (Vol. 1), S. Nonell, C. Flors (Eds.), European Society for Photobiology 2016. The Royal Society of Chemistry, Cambridge, UK.
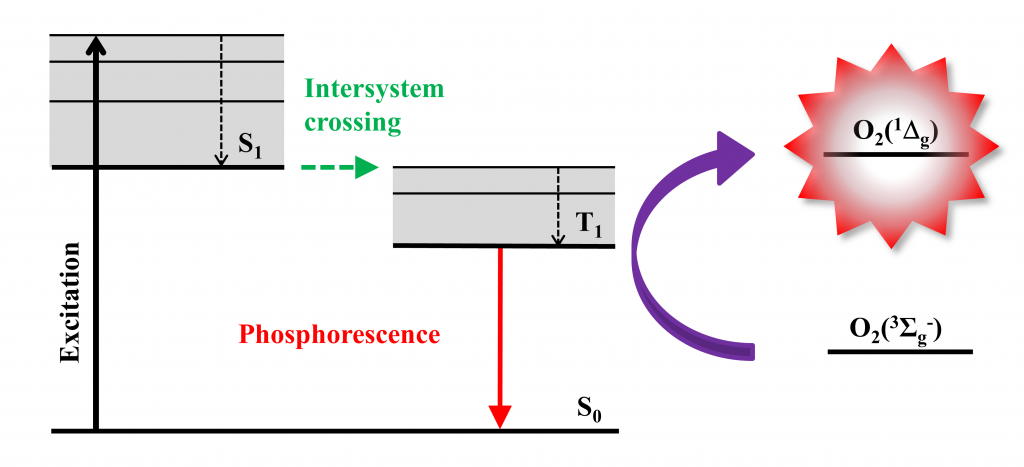
Figure 2. Production of singlet oxygen by energy transfer from the excited triplet states of a photosensitizer to molecular oxygen. Typical photosensitizers: octahedral molybdenum or tungsten clusters, porphyrins, phthalocyanines.
Clusters as luminescent and radioluminescent materials
Tetranuclear copper(I) iodide complexes with the cubane-like structure (Fig. 1A) have been investigated extensively due to their peculiar photoluminescence properties. As these complexes have affordable synthetic protocols, high photoluminescence yields, and are built from high Z elements, we investigated their radioluminescent properties under X-ray irradiation and suggested that these complexes with the {Cu4I4} cluster core are suitable for the design of scintillating materials (Fig. 3).
Selected reference:
Kirakci, K. Fejfarová, J. Martinčík, M. Nikl, K. Lang: Tetranuclear copper(I) iodide complexes: A new class of X-ray phosphors. Inorg. Chem. 56 (2017) 4610 – 4615.
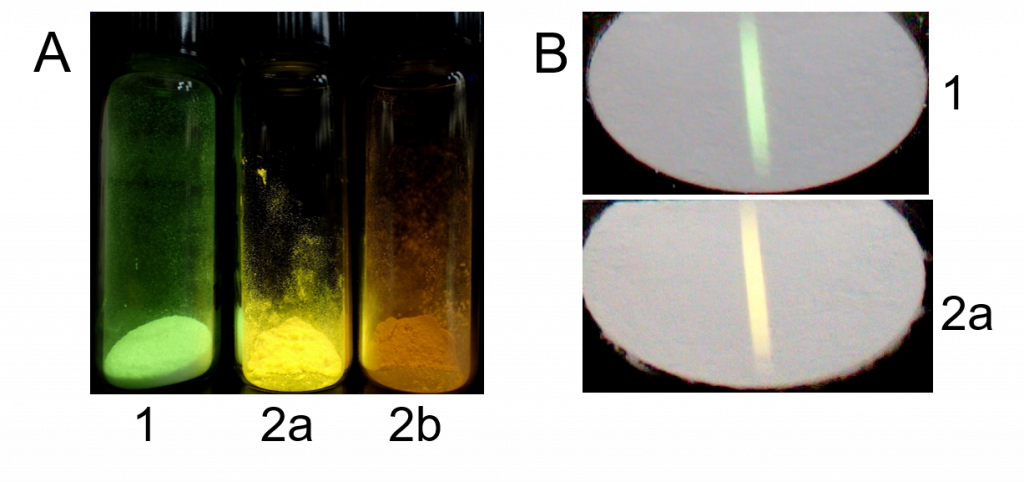
Figure 3. Photoluminescence of some Cu(I) complexes under 365 nm excitation (A) compared with their radioluminescence under X-rays (CuKα, 40 kV, 30 mA) (B
Clusters as photosensitizers and radiosensitizers of singlet oxygen
The octahedral molybdenum or tungsten clusters (M6) are nanometer sized metallic aggregates of Mo(II) or W(II) atoms stabilized by eight strongly bonded inner ligands (Li), usually halogen atoms, and six labile inorganic/organic apical ligands (La) (Fig. 4, see also Fig. 1B). Upon excitation with X-rays or light from the UV to green spectral region, these complexes form long-lived triplet states that relax via red-NIR luminescence with high quantum yields. This luminescence is efficiently quenched by oxygen, leading to the formation of O2(1Dg) in high yields. In contrast to commonly used organic photosensitizers such as porphyrins or phtalocyanins, which lose their photosensitizing activity upon aggregation, these complexes remain good O2(1Dg) photosensitizers even in their aggregated form. The high versatility, due to the choice of apical ligands, allows for using these complexes in their molecular form or to incorporate them into nanomaterials towards biological applications.
Photosensitizers such as M6 activated by light can be used in dermatology in the frame of photodynamic therapy (PDT). These photosensitizers can be also employed in the photoinactivation of antibiotic-resistant bacteria. Moreover, these clusters can be activated by X-rays in the frame of X-ray induced photodynamic therapy of cancer (Fig. 5). Indeed, X-rays have the advantage to penetrate deeply into tissues and can reach tumours that are not accessible to visible or infrared light. Furthermore, related photo/radioluminescence can be employed for diagnosis or analytical purposes.
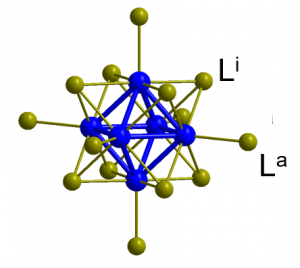
Figure 4. Structure of octahedral molybdenum or tungsten clusters (M6). Metal atoms are in blue, Li are inner ligands, usually halogen atoms, and La are labile inorganic/organic apical ligands.
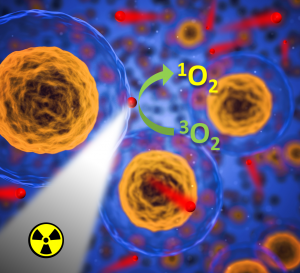
Figure 5. Schematic function of M6 nanoparticles (red ball) in X-ray induced photodynamic therapy of cancer.
Selected references:
K. Kirakci, J. Zelenka, M. Rumlová, J. Cvačka, T. Ruml, K. Lang: Cationic octahedral molybdenum cluster complexes functionalized with mitochondria-targeting ligands: photodynamic anticancer and antibacterial activity. Biomater. Sci. 7 (2019) 1386 – 1392.
K. Kirakci, J. Zelenka, M. Rumlová, J. Martinčík, M. Nikl, T. Ruml, K. Lang: Octahedral molybdenum clusters as radiosensitizers for X-ray induced photodynamic therapy. J. Mater. Chem. B 6 (2018) 4301 – 4307.
K. Kirakci, P. Kubát, K. Fejfarová, J. Martinčík, M. Nikl, K. Lang: X-ray-inducible luminescence and singlet oxygen sensitization by an octahedral molybdenum cluster compound: A new class of nanoscintillators. Inorg. Chem. 55 (2016) 803−809.
K. Kirakci, P. Kubát, J. Langmaier, T. Polívka, M. Fuciman, K. Fejfarová, K. Lang: A comparative study of the redox and excited state properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalton Trans. 42 (2013) 7224-7232.
K. Kirakci, P. Kubát, M. Dušek, K. Fejfarová, V. Šícha, J. Mosinger, K. Lang: A Highly luminescent hexanuclear molybdenum cluster: a promising candidate toward photoactive materials. Eur. J. Inorg. Chem. (2012) 3107-3111.
Clusters as radiocontrast compounds
Octahedral rhenium cluster complexes, e.g., [{Re6Q8}(CN)6]4–, where Q is S, Se, or Te, have very low toxicity, low luminescence quantum yields, however, they and attractive as radiocontrast agents.
Selected reference:
A. Ivanov, D. I. Konovalov, T. N. Pozmogova, A. O. Solovieva, A. R. Melnikov, K. A. Brylev, N. V. Kuratieva, V. V. Yanshole, K. Kirakci, K. Lang, S. N. Cheltygmasheva, N. Kitamura, L. V. Shestopalova, Y. V. Mironov, M. A. Shestopalov: The water-soluble Re6-clusters with aromatic phosphine ligands – from synthesis to potential biomedical applications. Inorg. Chem. Front. 6 (2019) 882-892.