Activated Borane
Contact person: Jan Demel
Activated Borane as a Promising Catalyst for Dehalogenation
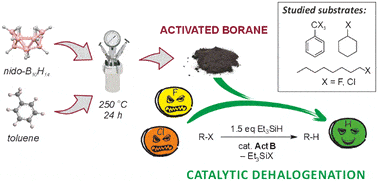
Activated borane (ActB) is a porous borane cluster polymer characterized by strong Lewis acidity. This material has been successfully employed as a heterogeneous catalyst for the hydrodehalogenation of aliphatic fluorides and chlorides using triethylsilane as a reductant. ActB exhibits high thermal stability, can be reused multiple times, and achieves exceptional catalytic activity with a turnover number (TON) of up to 5190.
Significance and Challenges
Organic halides are widely used in industry, but their strong C–Cl and C–F bonds make many of them highly persistent and potentially toxic. Dehalogenation thus becomes a key technology for the ecological disposal of these compounds. Traditionally, transition metal-based catalysts have been used for dehalogenation, but they are costly and environmentally burdensome. An alternative is the use of boron-based Lewis acids, among which ActB stands out due to its efficiency and the absence of metals or halogens.
Mechanism and Advantages of ActB
Activated borane is prepared by thermolysis of decaborane(14) in an organic solvent, forming a porous polymer. The catalytic mechanism of ActB involves the activation of the Si–H bond in triethylsilane, enabling the removal of halogen from the organohalide and its transformation into the corresponding hydrocarbon. ActB demonstrates remarkable stability and resistance to high temperatures, allowing for its repeated use without loss of activity.
Experimental Results
Experimental tests demonstrated that ActB efficiently dehalogenates various types of organohalides, including aliphatic, cyclic, and benzylic substrates. In the defluorination of trifluorotoluene (PhCF3), complete conversion was achieved after 1 hour at 180 °C. With cyclohexyl fluoride, the reaction was even faster, completing at 100 °C with almost complete conversion to cyclohexene.
Importance for Sustainable Chemistry
One of the most significant benefits of ActB is its ability to safely decompose persistent fluorinated compounds without using metal-based catalysts. ActB can be regenerated and reused, making it attractive for industrial applications, especially in the disposal of halogenated waste materials.
Activated borane thus represents a modern and sustainable solution for dehalogenation, combining high efficiency with environmental safety.
References:
- Catal. Sci. Technol., 2024,14, 4458-4465.
Borenium Salts
Contact person: Karel Škoch
Transition metals hold a prominent position in synthetic chemistry as catalysts for a wide range of chemical transformations, finding common use in both laboratory and industrial processes. However, their utilization is accompanied by inherent problems such as their high cost, significant toxicity, and environmental burden during their acquisition and purification. Especially in recent times, strategic risks are increasing as they are often obtained from politically problematic countries. For these reasons, there is a need to search for new and alternative approaches that would replace transition metals or bring new possibilities for synthetic chemistry.
Within our group, we are focusing on boron compounds carrying a positive charge, known as borenium salts. Typical compounds of tri-coordinated boron are archetypal Lewis acids and, as such, find application as electrophilic reagents, catalysts, or even as components of molecular sensors. By formally introducing a positive charge, there is a significant increase in electrophilic character, allowing these compounds to activate less reactive compounds and thus enter reactions that would not normally occur. Our goal is to better understand these compounds and their reactivity, and subsequently explore their use as catalysts for new and unusual chemical reactions.
In the course of our research, we have shown that some of these salts also exhibit interesting photophysical properties after UV irradiation, thus representing a unique type of Lewis acidic luminophores. By studying the influence of structure on photophysical properties, we have been able to describe a new class of compounds capable of modulating the emission maximum (i.e., the color of emission) achieving high quantum yields of luminescence. Thus, our interest is also extended to the chemistry of photoactive functional materials.